Chemical Reactions
| Topic Review on "Title": |
Chemical reactions are the “sentence” that shows a chemical change.
Components of a chemical reaction
Chemical reactions are made of reactants are listed first, followed by an arrow that indicates “yields,” “produces,” or “forms.” The arrow is followed by the products of the chemical reaction. Chemical reactions can also show states of matter and energy changes.
Common types of chemical reactions
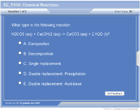
There are several common types of chemical reactions. Composition reactions are when more than one type of matter combines to form one molecule. Decomposition reactions are the opposite of composition reactions. Single replacement reactions involve and element reacting with a compound and replacing one of the elements in the compound. A double replacement reaction has two compounds that switch ions. A neutralization reaction is a double replacement reaction between an acid and a base. Precipitation reactions are double replacement reactions that produce an insoluble compound. Redox reactions involve the transfer of electrons from one atom to another, resulting in the change of an oxidation number.
Double replacement reactions and precipitations
Solubility rules can be used to determine if a double replacement reaction forms a precipitate, an insoluble ionic compound.
|
| Rapid Study Kit for "Title": |
| Flash Movie |
Flash Game |
Flash Card |
| Core Concept Tutorial |
Problem Solving Drill |
Review Cheat Sheet |
 |
 |
 |
|
| "Title" Tutorial Summary : |
Chemical reactions are the “sentences” of chemistry that show what molecules enter a chemical change and what molecules are produced in the change. The tutorial will introduce the components of a chemical reaction, the common types of chemical reactions and how to predict products of simple chemical reactions
|
| Tutorial Features: |
Specific Tutorial Features:
- Molecular animations of chemical reactions
Animations demonstrating how to determine products of a chemical reaction
Series Features:
- Concept map showing inter-connections of new concepts in this tutorial and those previously introduced.
- Definition slides introduce terms as they are needed.
- Visual representation of concepts
- Animated examples—worked out step by step
- A concise summary is given at the conclusion of the tutorial.
|
| "Title" Topic List: |
- Components of a chemical reaction
- Common types of chemical reactions
- Composition
- Decomposition
- Single replacement
- Double replacement
- Neutralization
- Precipitation
- Redox
- Determine products of a double replacement reaction
- Using solubility rules to determine a precipitate
|
See all 24 lessons in college chemistry, including concept tutorials, problem drills and cheat sheets:
Teach Yourself SAT Chemistry Visually in 24 Hours |



