Matter is made of atoms, which form elements and molecules.
Atoms
Atoms are the building block of matter. They are the smallest particle that retains the chemical properties of the element. Atoms are composed of protons, neutrons and electrons. Protons and neutrons are in the nucleus and contribute to the mass of the atom. Electrons are outside the nucleus, and together with the protons, contribute to the charge of the atom.
Ions
Ions are atoms that have a charge. There is an unequal number of positive protons and negative electrons.
Element Symbols
Element symbols are found on the periodic table. Element symbols can give lots of information: 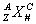 where X is the symbol, A is the mass number (protons + neutrons), Z is the atomic number (protons), C is the charge (protons – electrons) and N is the number of atoms present.
where X is the symbol, A is the mass number (protons + neutrons), Z is the atomic number (protons), C is the charge (protons – electrons) and N is the number of atoms present.
Isotopes
Isotopes are elements of the same atom (same number of protons) with a different number of neutrons (and therefore a different mass). The atomic mass found on the periodic table is a weighted average of all the isotope’s individual masses. The mass number shown in the element symbol above refers only to 1 specific isotope.
Atoms, Elements and Molecules
Atoms are the smallest particle retaining the chemical properties of the element. Elements are pure substances that contain atoms with the same number of protons. Molecules are pure substances that contain more than one type of atom chemically bonded together.



