Electrochemistry studies how chemical and electrical energy are converted.
Oxidation numbers & Redox reactions
Oxidation numbers are the charge on an atom if the electrons involved in the bond are assigned to the more electronegative atom in the bond. When oxidation numbers change during a chemical reaction, it is a redox reaction.
Oxidation number method of balancing
The oxidation number method is used for balancing simple redox reactions that cannot be easily balanced by the inspection method. It includes:
- Determine the oxidation numbers of each atom.
- Determine the net change in charge. Use the net change to determine the ratio of atoms that would cancel out the net charge change.
- Use the ratio as coefficients in the simplest compounds containing those elements.
- Finish balancing by the inspection method.
Half-reaction method of balancing
The half-reaction method is for the most difficult redox reactions:
- Use oxidation numbers to determine what’s oxidized and what’s reduced.
- Write two half-reactions, one for reduction and one for oxidation
- Balance all elements except H and O using inspection method.
- For an acid redox reaction: Balance the O’s by adding H2O to the side needing more O. For a base redox reaction: Balance O by adding twice as many OH- to the side needing more O.
- For a acid redox reaction: Balance the H’s by adding H+ to the side needing more H’s. For a base redox reaction: Balance H’s by adding H2O to the side needing more H’s.
- Determine the charge of each side of each reaction. Balance the charges by adding electrons to the side with the more positive charge for each reaction.
- Multiply the half-reactions by factors that will allow the electrons to cancel out.
- Add the two half-reactions back together.
Voltaic cells
A voltaic cell separates the reduction and oxidation reaction and forces the electrons to flow over a wire (producing electricity) from the oxidation reaction (at the anode) to the reduction reaction (at the cathode). The cell consists of the two separate half reaction, metal electrodes and a wire for conducting the electrons, and a salt bridge for balancing the charge build-up to extend the time the cell will operate. Line notation is a short-hand way of describing a cell:
- Anode written first
- Reactants written 1st on each side
- Anode & Cathode separated with ║
- Different states of matter within same side separated with │
- Same states of matter within same side separated with a comma
Cell potentials
The cell potential (or electromotive force) of a voltaic cell is due to the potential energy difference of the electrons before the transfer and after the transfer. A standard reduction potential is the potential that would be produced between a given half-reaction and hydrogen (hydrogen’s standard reduction potential has been defined as 0). The standard reduction potentials can be used to calculate the cell potential: EMF = cathode – anode. Positive EMF values indicate a spontaneous process.
Electrolytic cells
An electrolytic cell is the opposite of a voltaic cell. An electrolytic cell converts electrical energy into chemical energy by forcing a reaction to proceed in the non-spontaneous direction by putting electricity in. The voltage need to force the reaction in the opposite direction is at least that produced by the spontaneous process.
Electrochemistry and free energy
The free energy of a system can be defined as the amount of work that can be done by the system. The flow of electrons can do work. Therefore, the free energy of the system can be defined as: 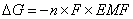 DG = free energy (in J); n = # of moles of electrons transferred; F = 1 Faraday; EMF = cell potential.
DG = free energy (in J); n = # of moles of electrons transferred; F = 1 Faraday; EMF = cell potential.
Electrochemistry and equilibrium
When a cell reaches equilibrium, the cell stops reacting.
The Nernst equation relates EMF to equilibrium:
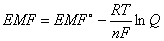 EMF = cell potential at current conditions; EMF° = cell potential at standard state (1 atm & 25°C); R = 8.31 J/mole´K; T = temperature (in Kelvin); n = moles electrons transferred; F = 1 Faraday; Q = reaction quotient
EMF = cell potential at current conditions; EMF° = cell potential at standard state (1 atm & 25°C); R = 8.31 J/mole´K; T = temperature (in Kelvin); n = moles electrons transferred; F = 1 Faraday; Q = reaction quotient
A cell stops when it reaches equilibrium. At equilibrium, EMF = 0 and Q = K 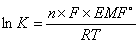 where K = equilibrium constant
where K = equilibrium constant



