Atoms and Elements:
- Atoms form bonds by gaining, losing, or sharing electrons. Bonding occurs when it produces a more stable electron arrangement.
- Covalent bonds are formed when atoms share electrons. They are very strong bonds, and are the major type in organic chemicals.
- Atoms become ions when they gain or lose electrons; ionic bonds are weaker than covalent bonds and tend to dissociate in water.
- Hydrogen bonds are weak intra- or inter-molecular attractions between molecules with a net dipole. Unequal sharing of electrons between H and an electronegative atom, such as O or N, creates dipoles.
Organic Molecules:
Molecular formulas express the composition of a substance in the simplest whole-number terms. Structural formulas describe how atoms are arranged in a molecule.
Stereoisomers have the same atoms, same order, and different 3D arrangement.
enantiomers: non-superimposable mirror images
diastereomers: not mirror images
Organic chemicals bonds:
- In single bonds, atoms share one pair of electrons.
- In
double bonds, atoms share two pairs of electrons.
- In triple bonds, atoms share three
pairs of electrons.
Common functional groups:
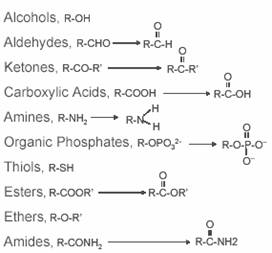
Important small organic molecules:
- Monosaccharides contain C, H, and O (1:2:1). Provide energy via metabolism (Glycolysis, Krebs cycle).
- Amino acids R-group specifies the identity (20 standard) as well as H20 solubility. Amide bonding forms peptides and proteins (e.g., enzymes).
- Fatty acids are hydrocarbon chain plus a carboxyl group. Forms ester bonds with glycerol to make glycerides .
- Nucleotides are polymers which function as genetic material: DNA=phosphate(s), base (G,T,A,C), deoxyribose RNA=phosphate(s), base (G,U,A,C), ribose.
Buffer Solutions:
Buffers are solutions of weak acids or weak bases and their salts (i.e., the buffer pair).
Buffers resist changes in pH upon addition of small amounts of acid or base.
The buffer pair HCO3_/H2CO3 helps maintain the blood pH around 7.4.
Biochemical Reactions:
-
The first law: the total energy of the universe is always conserved; energy can be neither created nor destroyed.
- The second law: the universe tends towards maximum disorder; all spontaneous processes occur in the direction that increases the entropy of the system plus its surroundings.
Entropy (S) describes the degree of disorder in a system. It increases with increasing disorder.
∆G - Gibbs Free Energy
∆G is the net change in free energy (products – reactants), given as kcal/mol or kJ/mol.
∆G = ∆H - T ∆S
∆H = enthalpy change, (>0=endo-, <0=exothermic)
∆S = entropy change T = temperature (K)
Enzymes in Biochemical Reactions
- Energy of activation (Ea) = the free energy necessary to start a reaction.
- Enzymes act as catalysts to lower Ea, but they do not change ∆G.
- Enzyme activity:
- Some enzymes are made in an inactive zymogen form and must be activated.
- Increase [substrate] and [enzyme] will increase the reaction rate until all the enzyme’s active sites are filled (Vmax).
- Increase T increases reaction rate to a point; excessive T denatures enzymes.
- Enzymes function best at certain pHs; excessive changes inactivates them.
- Allosteric effects and inhibitors regulate enzymatic activity.



